Top Fertility Specialists




You deserve to become a parent, no matter the odds. For the last 28 years, RSMC has strived to provide all the resources and care you need to start or grow your family. Our objective is to deliver quality fertility services to our patients around the globe with the utmost professionalism and compassion.


















Your new patient forms have been submitted and received. We look forward to seeing you at your appointment.
Please complete the form so we can best serve and help you with your journey towards parenthood.
You are all set and good to move forward. Focus on the treatment program and let’s bring your baby to you.
Based on the findings you can choose the right treatment package and payment plan including insurance program authorization, paying fully, or selecting the installment contract.
You can bring your card along when visiting for consultation or take a nice and clean pic of the insurance card and send it over email, fax, or through your patient portal.
Simply follow this link or email to info@fertile.com or or call at 858-436-7186.
You will travel to our San Diego location (all expenses covered) for retrieval. Eggs are retrieved vaginally through a 15-minute minimally invasive procedure under sedation. You will return home between 24-48 hours after your retrieval
Testosterone causes significant changes in the body. These can include stopping menstrual cycles and egg production. If you have already begun a hormonal transition, you need to stop testosterone to allow the eggs in your ovaries to develop again. The return of normal menstrual cycles suggests that the ovaries have resumed their normal ovulatory function. While it is possible to restore fertility after stopping testosterone intake, there isn’t a 100% guarantee. Transgender men who still have a womb can carry a pregnancy to term but will need to go off testosterone because it can inhibit the growth of a developing baby.
Medications used to prevent the body’s own release of LH and FSH from the pituitary gland (see Table 2). Note: The GnRH agonists (Lupron) can also be used to induce a flare-up or LH “surge.”
You will travel to our San Diego location (all expenses covered) for retrieval. Eggs are retrieved vaginally through a 15-minute minimally invasive procedure under sedation. You will return home between 24-48 hours after your retrieval.
Both you and the Intended Parents will have attorneys appointed to discuss agreements and create your contracts. If the Lucina Egg Bank selects you as a banking donor, you will create a contract directly with Physician’s Surrogacy which is the partner surrogacy agency of RSMC.
Once you are matched with either Lucina’s Egg Bank or an Intended Parent, we will conduct a comprehensive medical screening and psychological evaluation.
We will create a profile that highlights your most extraordinary qualities. We will present your profile toprospective Intended Parents and the Lucina egg banking committee.
“I have a whole new appreciation to give to surrogates and surrogacy san diego as a whole because of this agency. I gave birth to twins Nov 9, 2015 because of this agency and couldn’t be more thrilled with them all! The staff is wonderful, patient, caring, and they’re there to help every step of the way. They make sure you get everything you deserve and have all of the support you could ever want or need. The support group meetings are always fun and filled with food and I was so happy with this agency and I know if anyone looking for a potential agency looks into Physician’s Surrogacy which is the partner surrogacy agency of RSMC, they won’t be let down!! Thank you, RSMC for everything you did to help me complete a family.”
“In my quest to fulfill my dream of helping a family though surrogacy I researched several agencies in the California area, I found so many great reviews on Physician’s Surrogacy which is the partner surrogacy agency of RSMC. I called and the staff was quick to answer all my questions. They certainly helped put my mind at ease and was the first agency (I called several) that I spoke with where I finally sensed “this is where I belong.” I immediately felt like they cared for my best interested and walked me through every step pf the matching process, no question of mine was ever too big or too small. Within just a couple of months of my profile being listed I had a conference call with a potential set of intended parents. A short time later, it was a match! It just took one phone call and I knew that all my wishes in what I was looking for in a set of intended parents had been listened to and met. Not long after, I had to travel for my initial medical clearance, surrogacy agency made all my travel arrangements and were in constant contact with me. Not quite
a year after my matching process and I am quickly nearing delivery of my first surrogate baby. The entire staff at the surrogacy agency has helped make this the most positive, joyful experience I could’ve ever imagined. If I ever need anything, my coordinator is quick to get on it and be sure it’s taken care of. I have gotten phone calls and texts from the staff congratulating me on important milestones (heartbeat confirmation, 20-week ultrasound, first, second and third trimester, etc.) surrogacy agency has always made me feel like family, I couldn’t have asked for a more pleasant experience!”
“Physician’s Surrogacy is an amazing agency. As a first-time surrogate, I knew that helping someone have a family was something I wanted to do, however, I had no idea how life changing this would be. I was matched with a wonderful couple — it could not have been any better of a match. For this couple, I carried twins, a boy, and a girl. Throughout my pregnancy, I felt supported, cared for, and appreciated by my IPs and everyone at the surrogacy agency. When I look back on my experience, I realize that I have given a couple a beautiful family, gained a good friend (Mom), and developed a new level of closeness with my husband. I am changed forever in a beautiful way!”
“Successful journey! So happy I was able to deliver this healthy baby boy for his family. I completed my first surrogate journey & the process went smoothly & my case manager Erika was always there when I needed her! If you’re looking for a surrogate agency, I would highly suggest Physician’s Surrogacy which is the partner surrogacy agency of RSMC. I have had no issues and I am so thankful for the medical team & the staff that helped me & the intended parents.”
Surrogate and Intended Parents are matched based on detailed criteria. A comprehensive legal contract is signed with clear legal guidelines for each party
You will travel to our San Diego location with all necessary expenses covered. One of our skilled physicians will retrieve the eggs vaginally through a minimally invasive procedure.
我今年大三,因為從小就喜歡美國文化,所以大學念了外文系,更計畫在畢業前環遊美國。但無奈旅費是一筆很大的數目,即使我平日兼家教,還是無法達標。偶然間上網發現許多美國的華裔父母需要愛心捐卵人的幫助,於是看了許多人的推薦,最後才選擇風評最好的RSMC醫療生育中心。
由於這是我的第一次捐卵,RSMC的專業人員,詳細為我解釋整個流程和用藥注意事項,才知道原來捐卵是取女性身體未使用剩餘的卵子,而不是網路上謠傳的如此傷身。最後順利到美國完成捐卵,我的身體恢復得很快,經過檢查後,隔天就飛到東岸,開始環遊美國之旅,最令我感到貼心的,是RSMC的員工姊姊,知道我要去旅遊不但推薦許多景點,還熱心告訴我旅行要注意的事項,更留下她的電話,叮嚀我若遇到狀況,可以打電話請她幫忙!滿滿的人情味,讓我不得不推薦RSMC !
我的姊姊因為卵巢早衰一直無法孕育孩子,做過許多試管嬰兒療程都失敗,見她流過多少淚,多少的辛酸不為人知,所以我很能辛苦體會不孕家庭的痛楚,下定決心捐卵,幫助和她一樣的媽媽。
為此我上網做了許多功課,發現許多捐卵仲介介紹的醫院環境和環境都十分糟糕,只有RSMC是美國合法醫療中心,擁有專業的醫護人員。過程十分順利,手術只有短短不到20分鐘,快得讓我都不知道取卵已經完成。醫生說我的卵子十分健康,質量也很好。經過這次經驗,也許有一天我也能捐卵給姊姊,幫助她擁有孩子,那將會是很美好的一件事!
兩年前,我曾經捐過卵,當時是朋友介紹的仲介阿姨,一開始人非常熱心,詳細地幫我解答很多問題,直到幫我找到一家所謂的捐卵醫院後,人就開始消失,不回覆訊息,之後就是惡夢地開始,過程中許多細節我都沒有人引導,也沒有專業醫護人員詢問,所幸最後完成捐卵,身體也沒有大礙;不過這次不愉快經驗,也讓我決定不找仲介。
經過朋友的推薦,第二次我選擇了RSMC醫療中心,這次RSMC的專業協調員和醫護人員,仔細解答所有流程,甚至比我更擔心我的健康安全,叮嚀我所有注意事項,給像家一樣的溫暖感受,這此取卵結果非常成功,術後我也沒有任何不適!我已經預約我的下一次取卵,就在今年5月,希望我的貢獻,可以造福更多家庭。
我今年20歲,即將大學畢業,但想到沉重的學貸還沒付完,心頭就感到徬徨無助。還好,偶然發現RSMC徵愛心捐卵天使,便上網填寫申請表格,很快速地得到匹配。
一開始我對捐卵一無所知,但RSMC專業的醫護人員,在過程中耐心地解答每個疑問,甚至關心我的身體狀況;他們專業認真的態度,讓我備感安心;最後順利地完成捐卵,取出了多顆健康的卵子,才發現原來這一切比想像地還要簡單輕鬆!
更重要的是,這筆愛心回饋金幫助我還清剩下的學貸,一對美國的華裔夫妻,在我的幫助下獲得了一對雙胞胎。幫助人之餘,還能獲得豐富的報酬,沒有什麼事比這還令我感到開心且值得!
“It breaks my heart to know some people are unable to have a child of their own. I became a donor because helping someone have a child is an amazing thing. I wanted to give a part of me (that I luckily have) to someone else and change their lives.”
“I know there are couples out there that can’t have children and I’ve seen firsthand how devastating that can be, seeing my best friend go through her lowest point in life when she found out she couldn’t have children due to health issues. I believe everyone has a purpose in life and I strongly believe this was my purpose. There would be no greater feeling than giving someone a chance at a family, something that many of us take for granted.”
“I wanted to become an egg donor for many reasons. First, my passion for health leads me to admire the ability of the human body to reproduce. I am currently taking an embryology course and I find all of it so fascinating! I believe it would be an incredible honor to help someone who could not otherwise have a child fulfill their dreams of having one. This knowledge alone is a huge benefit to me! And any compensation helped me pursue my own dreams of becoming a women’s health specialist by helping fund my current medical school education.”
If you meet the pre-qualifications you will be asked to complete part 2 of the application, which will ask in-depth questions about your personality traits, family medical history, and more.
Create an account and complete the pre-qualification application to find out if you qualify. You will find out instantly whether you can be accepted. This application is not a commitment, it’s a simple way to find out if you’re eligible to become an egg donor.
Currently the infertility industry is segmented and each treatment or service is handled by a different provider. Our full-integrated model streamlines an otherwise complicated process. We exist to revolutionize the industry by providing a one-stop-service model to help our patients navigate through the infertility maze, minimizing financial, physical and emotional risks.
The surrogacy agency is fully transparent about the pricing of procedures and any additional costs that may occur before your treatment begins. Some clinics may lower prices before the contract signing but charge more at a later date. We make it our priority that our patients are aware of any potential additional pricing that may occur during the treatment.
Our clinic focuses on family building options regardless of your sexual orientation or the gender you identify yourself. We are actively taking additional steps to make members of the LGBT community feel welcome at our clinic. All of our patient-facing staff undergoes LGBT training for family-building clinicians to better support and make you feel welcome.
Our internationally respected team of experts are advancing IVF technologies to enter into a future generation of better outcomes for infertility. While you will have one physician guiding you, you will also benefit from the combined experience and insights of our physicians during weekly case review collaboration meetings. That way you will not only be able to rely on the expertise of one fertility expert, but multiple renowned specialists.
Our team specializes in challenging cases and accept patients who may have been deemed “hopeless” at other clinics. Due to our customized solutions, expertise and full-range of internal collaboration, we are able to maximize pregnancy success rates that surpass the industry average even in complicated cases.
Every situation is unique and every body needs different treatments. Our fertility experts use over 40 customized protocols to increase success rates for our patients whereas many facilities take a “protocol fits all” approach.
The personalized approach even extends to our IVF lab. Our full-time laboratory director and lab team nurture each egg and embryo to support the maximum success of each and every cycle.
We believe that we have the most affordable pricing available in the area. Should you have received a better pricing quote from another local fertility clinic for the same treatment plan we will happily match the price.
Physician’s Surrogacy which is the partner surrogacy agency of RSMC is fully transparent about the pricing of procedures and any additional costs that may occur before your treatment begins. Some clinics may lower prices before the contract signing but charge more at a later date. We make it our priority that our patients are aware of any potential additional pricing that may occur during the treatment.
Our 3rd Generation IVF, also known as Preimplantation Genetic Diagnosis (PGD), is beyond exceptional with our very own specialist leading the medical team. We screen for and identify specific genetic diseases, eliminating inheritance in future generations.

The state-of the art laboratory accounts for up to 30-40% of success rates. Count on centralized control and strict oversight by an experienced, full-time lab director.

Physician’s Surrogacy which is the partner surrogacy agency of RSMC has a distinct advantage over other clinics. You’ll have a higher chance of success with our extensive and specialized protocols. We provide a level of customized care that considers many factors contributing to infertility and identifies the best next steps.

Everything you need is in-house, which makes your journey easier. We provide consistent communication and care while guiding you through the process.

Our expansive surrogacy program features a stringent screening and selection process. This ensures our surrogates are healthy and highly qualified to carry a pregnancy to term.

When it comes to your journey, our goal is to make it as smooth and seamless as possible. Our proprietary insurance plans cover third party-related medical expenses as well as unexpected situations.

A surrogate is a partner throughout your journey. Our surrogacy program has a strict screening and selection process so you can trust your surrogate is healthy and highly qualified to carry a pregnancy to term.
The path to parenthood is a physical and emotional journey. Count on qualified physicians and reproductive specialists to guide you through each step for a successful pregnancy.

You’ll have a higher chance of finding a perfect match with our egg donation program, which features diverse women of various backgrounds.

Feel confident knowing our doctors and embryologists have more than 150 years combined experience, resulting in high surrogate and egg donor pregnancy rates (80% and 87% respectively).
If IVF or IUI is not an option, RSMC helps you find a surrogate through our in-house, OB-managed surrogacy program. Through our program, all Surrogates have been pre-screened with our proprietary process, offering you peace of mind and confidence by ensuring their medical and emotional readiness to help you achieve your dream. Our program is less than 50% below the national average for pre-term delivery rates, maximizing the safety for both the Surrogate and your baby. An experienced physician will closely monitor the pregnancy along with your case manager. Detailed reports are sent directly to you on a weekly basis. Your Surrogate has a dedicated case manager and psychologist to support her throughout the journey.

Egg donation allows individuals and couples to experience parenthood by using another woman’s eggs. Utilizing donor eggs is a great option for women who have undergone treatment such as chemotherapy or radiation. After these treatments, women are unable to naturally reproduce using their own eggs. The process involves utilizing eggs from another woman and fertilizing them with sperm to create an embryo. The embryo is then implanted in the intended mother or surrogate’suterus via IVF.
Today, the power is in your hands to delay parenthood to pursue your career, educational or personal goals. Most women don’t realize there is a dramatic decline in egg quality during your 30s and fertility preservation puts you in control of your timeline. The process is similar to IVF, but instead of the embryos being created and transferred, they are cryopreserved and stored. Once you have discussed everything with your partner and standard fertility testing has been completed, your physician determines which option is best for you. We have made fertility preservation even more accessible by offering one of the most affordable egg freezing programs.

This option makes it possible for both you and you partner to have a biological connection to your child. It is the exact same process as IVF. However, the eggs are retrieved from one partner and the resulting embryos are transferred into the carrying mother’s uterus. Since you are going through the process together, fertility medications are used to sync your cycles. The woman who is providing the eggs goes through an ovarian stimulation cycle and the egg retrieval procedure.
IVF is another popular option for many situations, especially if you are using donor eggs. In the process of IVF, you/your partner/Egg Donor undergo ovarian stimulation to develop mature eggs which are then removed from the ovaries and fertilized in a laboratory. The resulting embryos are transferred into either your uterus, your partner’s uterus, or a Gestational Carrier if needed. Moreover, our IVF pricing is one of the most affordable and comprehensive ones in California and neighboring states.

Also referred to as artificial insemination, in which washed and prepared sperm is placed near the egg inside the uterus during an ovulation cycle. Fertility drugs are used to help track ovulation and increase your chances of success. In this case, your or your partner’s eggs and donor sperm will be used for embryo creation.
You’ll find your perfect match through our extensive donor database. Whether you are looking for a donor with high IQ, specific aesthetics, gifts or talents, our matching program assures you select the characteristics most important to you.
Your journey to parenthood will be shorter and more predictable because of our Egg Bank. Quality donor eggs are frozen through vitrification and stored in our on-site laboratory. Electing to use banked eggs puts you in control of your timeline, since they are available for immediate use. Additionally, selecting donors from our bank eliminates waiting time and unforeseen setbacks. You will maximize success rates due to a guaranteed yield of high quality, mature eggs.
Your journey to parenthood will be shorter and more predictable because of our Egg Bank. Quality donor eggs are frozen through vitrification and stored in our on-site laboratory. Electing to use bank to eggs puts you in control of your timeline, since they are available for immediate use. Additionally, selecting donors from our bank eliminates waiting time and unforeseen setbacks. You will maximize success rates due to a guaranteed yield of high quality, mature eggs.
The simplest option is natural conception through sexual intercourse. If you or your female partner is facing an infertility problem, your health insurance may cover the treatment costs.
If you are considering growing a family before undergoing hormone therapy and/or gender confirmation surgery, sperm banking is an essential step you need to take. If you choose to freeze your sperm after starting hormone therapy, you would require a short pause in therapy in order to produce a good specimen.
If your partner is another trans man, your frozen eggs can be inseminated with the donor’s sperm to produce embryos. The resulting embryo can then be transferred into a Surrogate who will help you carry the pregnancy
If you’re partnered with a trans woman, your frozen eggs can be inseminated with a donor’s or your partner’s sperm (if available) to produce embryos, which are then transferred into a Surrogate, who will carry the pregnancy
Your partner’s sperm can be used to fertilize your frozen eggs. You may also need a Surrogate to carry the pregnancy for you.
Before transitioning and starting testosterone therapy, there is an option to conceive through intrauterine insemination (IUI) using donor sperm.
If your partner is another trans man, your frozen eggs can be inseminated with the donor’s sperm to produce embryos. The resulting embryo can then be transferred into a Surrogate who will help you carry the pregnancy
If your partner is a cis woman, you can use your own frozen eggs to form embryos. These are then implanted into the womb of your partner, who will carry the baby to term (Reciprocal IVF/Partner Assisted IVF). You are going to need donor sperm in this case.
Egg freezing or oocyte preservation is an essential family-building option for transgender men who intend to use their own eggs to create a biologically related baby in the future. Since egg quality, egg quantity, and female fertility all decline with age, the younger you are when freezing your eggs, the higher the chance of successful conception in the future. Eggs can be frozen for as long as you want, and once they are frozen, the health of the eggs will remain stable without decreasing in quality over time. We have made egg freezing highly affordable for transgender couples and individuals.
Testosterone causes significant changes in the body. These can include stopping menstrual cycles and egg production. If you have already begun a hormonal transition, you need to stop testosterone to allow the eggs in your ovaries to develop again. The return of normal menstrual cycles suggests that the ovaries have resumed their normal ovulatory function. While it is possible to restore fertility after stopping testosterone intake, there isn’t a 100% guarantee. Transgender men who still have a womb can carry a pregnancy to term but will need to go off testosterone because it can inhibit the growth of a developing baby.
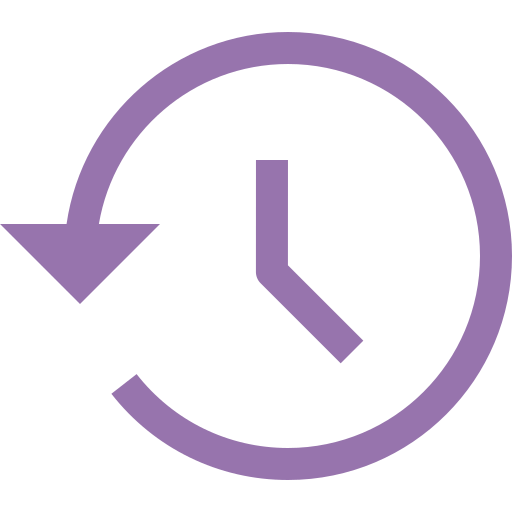
Our egg bank makes your journey to fatherhood shorter and less risky. It puts you in complete control of your timeline since banked eggs are available for immediate use. Selecting from our egg bank also eliminates waiting time and unforeseen setbacks. When you use our egg bank, you are guaranteed high-quality eggs, which maximizes success rates. Get Egg Bank Access.
You can also use the eggs of someone you know, such as a close friend or family member. You’ll already know your donor’s personality, and potentially their health and family background. They will also have the unique opportunity to be a part of your child’s life, which can be incredibly rewarding. A known donor will still be required to go through the same screening and retrieval process as an anonymous donor
If you decide to go with an anonymous donor, surrogacy agency has an extensive donor database to choose from. Our donors are thoroughly screened to ensure that they have high-quality and viable eggs. Our extensive database consists of a wide variety of donors worldwide, allowing you to select the Egg Donor that best suits your wants and needs.
There are a variety of hormones associated with reproduction. We typically test for levels of FHS, LH and AMH to determine your remaining egg supply. FHS levels are a good indicator of egg production. LH levels trigger ovulation and development of the corpus luteum. AMH indicates the presence of growing follicles. Hormone testing will also determine which partner has the highest quality eggs.
Excellent care and great support services! They spent a huge amount of time with me and my husband as we weighed different options. We got pregnant after transferring just one embryo! I can’t think of a single negative thing or reservation about recommending Physician’s Surrogacy which is the partner surrogacy agency of RSMC. Everything was great, which isn’t easy in what can be a difficult process.
I am enthusiastically offering this positive patient feedback on behalf of the doctors and amazing staff at Reproductive Sciences Medical Center in Del Mar. My wife and I dreamed of having children since we fell in love 13 years ago. When it was finally time, we tried and tried until we realized it just wasn’t going to work the old-fashioned way (though we had a lot of fun!)
We searched for the best assistance with our fertility issues and found it with surrogacy agency. We could not have been treated better throughout our journey, which can be very emotional at times. The care we received at the surrogacy agency made all the difference. In the end, we were blessed with a beautiful and healthy baby boy!
We couldn’t be more pleased with the surrogacy agency staff and our outcome! We are so thrilled to have 2 healthy babies from just 1 viable embryo! What a tribute to the extraordinary skills of the doctors and the whole surrogacy agency staff. We wouldn’t go anywhere else if we try again, and will continue to refer our friends and rave about our experience. Thank you for our miracle babies! Without you, our family wouldn’t be the same. We will forever be grateful!
Because I am Chinese and grew up in a medical family, I have used many Chinese therapies to try to overcome infertility issues. However, after 6 years of trying and numerous Chinese doctors (Acupuncture, herbs, etc.), there was still no success in sight. We went to another IVF clinic and did not get pregnant in the first cycle.
While seeing your reproductive endocrinologist, you discuss your efforts and failed attempts. Your doctor may draw blood, test and evaluate hormone levels, as well as collect a sperm sample from your partner. There are several tests and evaluations your doctor will use to determine infertility. (Infertility Diagnosis and Testing) Once lab results are back, your doctor will discuss the treatment options and next steps. While the road leading to this point may not have been easy, you are not alone.
Our medical team at the surrogacy agency will help you have the family of your dreams. We specialize in challenging cases and are dedicated to helping you achieve your family goals.
It’s been months, maybe a year, since you’ve started trying. Tracking your ovulation and planning accordingly has not been successful. You and your partner start to google reasons why you’re not able to conceive with the hope of finding a new method. You try the recommended methods, but the result is the same. Uneasiness sets in and you both decide it’s time to see a fertility specialist.
It’s been a couple of months and your period continues to be consistent and on time. You may start to wonder if your timing is off. During this phase, you may try to be more clever and start utilizing ovulation trackers to understand your fertility window.
Now that you know when you are ovulating, you plan accordingly, hoping for a positive result. Unfortunately, no signs of pregnancy occur. You start to become concerned and compare your situation to those of your friends and family who were able to conceive in the same amount of time.
After you and your partner have decided it’s time to start a family, you both stop using birth control and let nature take its course. At this time, you may not be tracking your fertility window and may be having sex whenever the mood is right.
Following the preimplantation genetic screening process, which makes sure there are normal numbers of chromosomes and diagnoses possible genetic disorders, the most viable, healthiest embryos are selected for freezing. Thanks to the development of vitrification also known as flash freezing, you have a better chance of having a successful pregnancy using frozen embryos. Vitrification enables over 90% of frozen embryos to survive the thawing process and prevents them from declining in quality. Your embryos are gently handled and securely stored in our on-site lab. Unlike other fertility laboratories, the surrogacy agency has a lab director that works full time, which shows our commitment to quality since more than 40 % of IVF success depends on the laboratory.
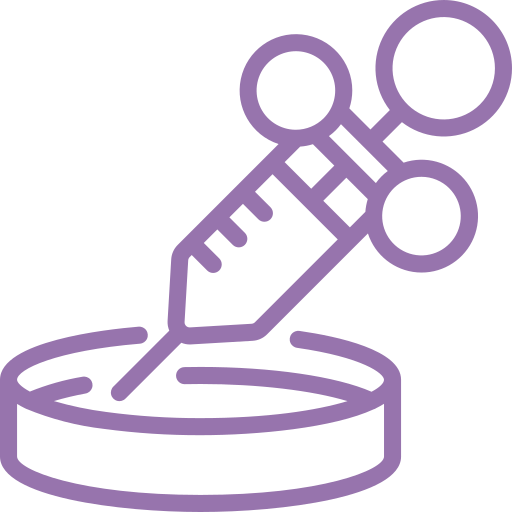
For fertilization to take place, the harvested egg and best-quality sperm are “mixed” and left in a Petri dish containing a culture media and then placed in an incubator. This dish is closely watched to check if fertilization has occurred. Once the eggs are fertilized, they are referred to as an embryo or a blastocyst on the 5th day of development. Our in-house embryologist will then nurture the embryos until they are ready to be implanted or frozen, even if that means working outside the standard operating time. For instance, if an oocyte is not yet mature, we will wait for it to achieve maturation and then ICSI it at the right time.
The egg retrieval is a minimally invasive, non-surgical procedure, that lasts approximately 20-30 minutes. You will be put to sleep by anesthesia for the procedure. Using ultrasound technology, your physician will retrieve the eggs with a fine, hollow needle attached to an ultrasound scan probe. Once the eggs are retrieved, the partner’s fresh or frozen sample of semen or donated sperm you have selected beforehand is washed.
The day after hatching, the embryo is transferred to the uterus where it will hopefully implant and result in a successful pregnancy.
The embryo is held with a specialized holding pipette. A very delicate, hollow needle is used to expel the acidic solution against the outer “shell” (zona pellucida) of the embryo. The acidic solution creates a small hole in the shell.
The collected sperm are examined and evaluated for their health, strength, and motility. The patient may choose to utilize the sperm to fertilize his partner’s or a donor’s egg or may also choose to preserve the sperm for later use.
The collected sperm samples are separated into multiple containers. Skilled lab technicians will use cryoprotectant, a substance used to protect the sperm from freezing damage. The frozen vials of sperm are stored in the lab until the patient is ready to use them
The male patient will provide a semen sample through masturbation. If the patient does not produce sperm in the ejaculation, a sperm sample may be taken from his testes involving MESA, a surgical retrieval.
When the embryos are ready for PGS and PGD testing, six to eight cells are extracted from the embryo (usually after day 5 or 6). This is called a blastocyst biopsy. During the biopsy, our laboratory technician uses a narrow biopsy pipette to remove cells from the outer layer of cells (trophectoderm). The embryologist will then freeze the embryo and wait for the test results.
Genetic testing is usually completed within one day of the embryo biopsy, which allows for a timely embryo transfer. During this step, chromosomes are inspected for genetic abnormalities.
After the egg has been fertilized, embryos are transferred into a fluid that allows them to develop for several days. On day three, the healthy embryos should have developed into eight cells.
The dish is placed in an incubator to allow for fertilization. After fertilization has taken place, the embryo is then transferred into the uterus or frozen for future implantation.
A hysteroscopy may also be used to determine if there is scarring, uterine fibroids, or polyps in a woman’s uterus. A long camera is inserted into the vagina and its feed appears on a monitor for examination. The turnaround time for this test is within a day.
This test is usually reserved for women over 35 who are more likely to have a low ovarian reserve. It is a series of blood and imaging tests that help determine the quality and quantity of eggs available for ovulation. The turnaround time for this test is within a day.
A sperm sample is taken from the man’s semen or is extracted from his testes if he has trouble ejaculating with sperm. Motile sperm are selected, prepared, and washed for insemination. Additionally, the woman’s eggs are surgically extracted from her ovaries. Mature eggs are chosen for injection.
Once ovulation occurs, the washed and selected sperm will be inserted using a thin catheter (tube) through your cervix and into the uterus. If ovulation doesn’t occur, you may be administered an HCG shot. Once the shot is administered, the insemination process will continue within 24 to 40 hours.
IUI typically takes place during a natural ovulation cycle without the use of fertility drugs. The procedure is done between day 12 and 16 of a woman’s menstrual cycle. In certain situations, fertility drugs may be used to help stimulate your ovaries and develop multiple mature eggs for fertilization. If needed, fertility drugs are typically administered at the beginning of a menstrual cycle.
A contrast dye is injected into the uterus and an X-ray is taken to detect abnormalities in the uterine cavity. This examination will determine whether there is a blockage in the fallopian tubes that prevents movement of the egg from the ovaries. The turnaround time for this test is within a day.
There are a variety of hormones associated with reproduction. We may test for levels of follicle stimulating hormone (FSH) and anti-mullerian hormone (AMH) to determine your remaining egg supply. High FSH may indicate ovarian failure or perimenopause. Low FSH levels may indicate reduced egg production, while AMH indicates the presence of growing follicles. The turnaround time for this test is within a day.
Once a donor is matched, the intended mother/Surrogate and the donor will undergo medical screening and start the cycle synchronization. You have to partner with a fertility clinic to complete this step. The surrogacy agency itself is a full service fertility center and has a reputable record of above national average success rate.
Both the intended mother/surrogate and the donor will undergo various medical and psychological screening. Once all the screenings are passed, the donor and recipient will start taking medications to synchronize their menstrual cycles.
The resulting embryos are transferred into the recipient’s womb. A blood test is done after two weeks to determine if the transfer was a success and pregnancy is achieved. The extra embryos can be frozen to be used later.
After the Preimplantation Genetic Screening (PGS) process, which ensures there are normal chromosome numbers and detects potential genetic disorders, the healthiest embryos are chosen to be inserted into your (or a chosen surrogate’s) uterus. Two weeks after the embryo transfer, your physician will take a final blood test to measure the level of your hCG (human chorionic gonadotropin) hormone. Elevated hCG levels typically suggest a positive pregnancy test.
For fertilization to occur, the retrieved egg and highest-quality sperm are combined and left in a dish to culture in an incubator. The dish is closely monitored to see if any of the eggs have been fertilized. Once fertilized, the egg becomes an embryo, also called a blastocyst on the fifth day of development. Our full-time on-site embryologists nurture each embryo at the appropriate time even if it falls outside of standard business operating hours. For example: If an oocyte is immature, our lab will wait for maturation and then perform ICSI of the oocyte at the appropriate time.
egg retrieval is a minimally invasive, non-surgical procedure, that lasts approximately 20-30 minutes. You will be put to sleep by anesthesia for the procedure. Using ultrasound technology, your physician will retrieve your eggs with a fine, hollow needle attached to an ultrasound scan probe. Once the eggs are retrieved, your partner’s fresh or frozen sample of semen or donated sperm you have selected beforehand is utilized for fertilization. The sperm are washed, and the best-quality sperm extracted will be used to fertilize the eggs.
Your physician will create a personalized medication schedule that details the necessary fertility drugs and hormone injections you will need. Medication and injections are administered to stimulate your ovaries to develop a greater number of mature eggs for fertilization. Each woman responds to fertility drugs and hormones differently, so customized protocols are essential to success. We will monitor you carefully, helping you understand the changes in your body, and keep track of how your follicles are developing.
You have a higher chance for a successful pregnancy with the development of the “flash freezing” process, known as vitrification. Until vitrification was introduced a decade ago, frozen embryos had a very low implantation and live birth rate. One of the primary issues was the freezing process itself. The slow freezing method enabled ice crystals to form within the embryo, which damaged it to the point at which they were not longer viable. Fortunately, with the development vitrification, more than 90% of the frozen embryos survive thawing and are the same quality as fresh embryos.
Your eggs are handled and stored securely in surrogacy’s agency on-site laboratory. Unlike other fertility labs, surrogacy agency has a full time Laboratory Director which reflects our commitment to quality as the lab accounts for over 40% of the overall success of IVF.
A transvaginal ultrasound aspiration is used to retrieve eggs from the ovaries. The procedure is a minimally invasive, non-surgical and always conducted under sedation. The procedure itself lasts 10-20 minutes.
Hormone injections are administered for 10-14 days in order to stimulate the ovaries, so you release more eggs. Normally our bodies release just one egg each month but with cryopreservation the goal is to retrieve as many healthy eggs as possible.
The consultation will be dedicated to enhancing your understanding and setting realistic goals. Your fertility doctor will perform a physical exam and initial fertility tests. After the tests have been evaluated, our expert medical team will move forward with creating a personalized plan.
Once your physician creates your plan of care, you will meet with a financial coordinator. The coordinator will review with you a summary of the health benefits obtained from your insurance provider. The coordinator will provide you with a detailed quotation based on the treatment plan outlined by your physician and will also discuss any financial questions you may have. If you have obtained other quotes, we recommend you bring them to the appointment, and your financial coordinator can review them with you to compare the proposed services and costs.
Your Reproductive Endocrinologist will consider all factors and develop a comprehensive plan of care, also known as your treatment plan. The plan includes the recommendations for treatment from the physician and allows your financial coordinator to create an accurate quotation once you meet.
All initial onsite consultations will include a fertility evaluation, which consists of a physical examination, follicular ultrasound and testing, allowing the physician to evaluate your current fertility status and create a comprehensive treatment plan. If you elect for an initial virtual consultation, your fertility specialist will schedule your fertility evaluation at a subsequent visit.
The consultation includes a comprehensive evaluation with your physician. Together, you will review your goals and medical records, allowing ample time to discuss your goals and questions. We encourage you to write down your questions before your visit to enable you to maximize the time spent with your physician.
Upon your arrival, you will check in with a Patient Care Coordinator.
Congratulations! You meet the prequalification criteria.
Click here to complete the full application and find out if you are accepted into our program.